EHR to EDC
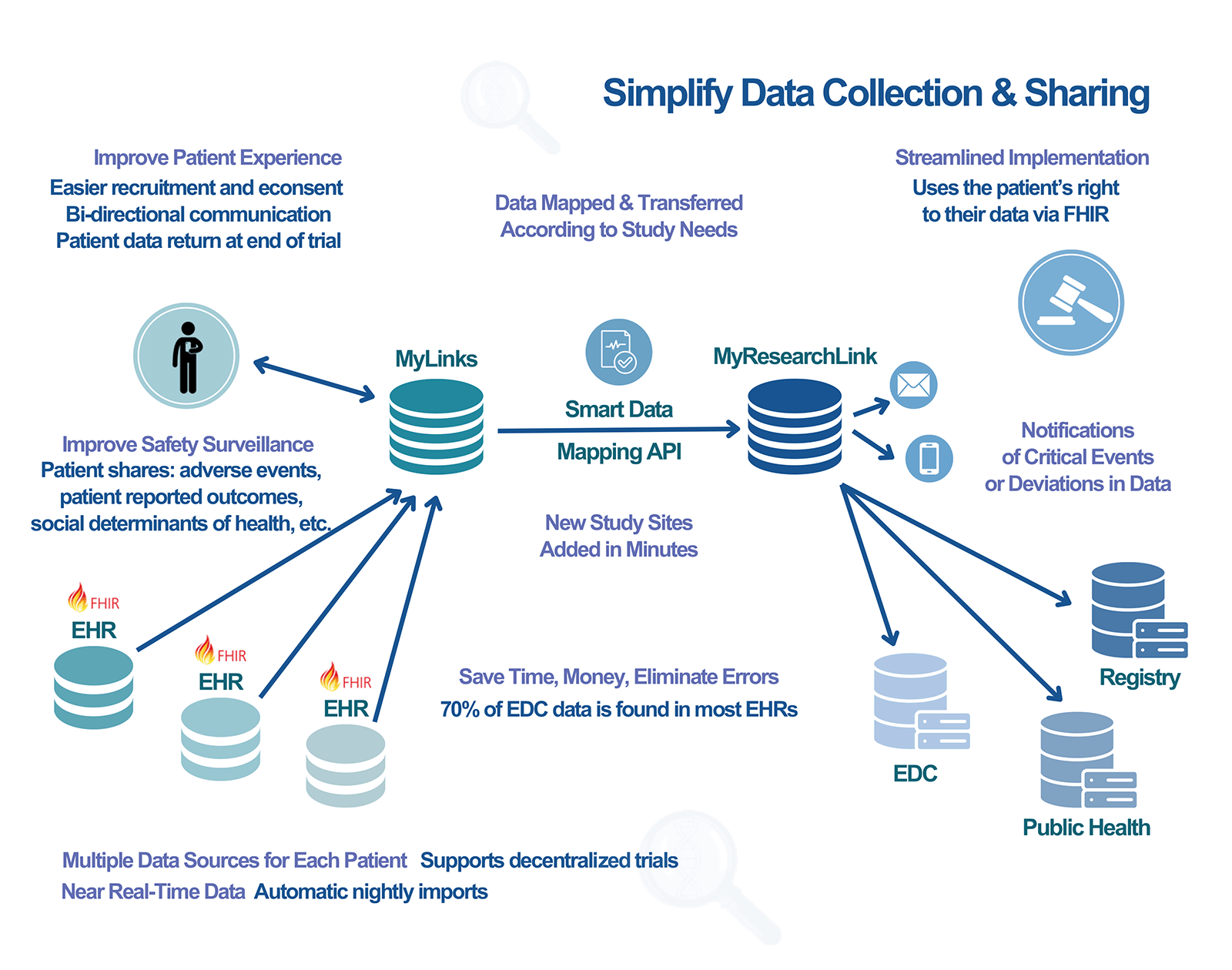
Data flows from the EHR to MyLinks then to ResearchLink with patient consent.

Seamlessly Transfer Patient Data from EHR to EDC with Our Patient-Facing FHIR App
Welcome to a new era of clinical trial data management. Our innovative patient-facing Fast Healthcare Interoperability Resources (FHIR) application empowers participants to securely transfer their electronic health record (EHR) data directly to the Electronic Data Capture (EDC) system, streamlining the research process and enhancing data accuracy. Join us in revolutionizing clinical research with patient-mediated data exchange.
Why Choose Patient Data Transfer via FHIR App?
Key Features of Our Patient-Facing FHIR App
Who Can Benefit?

